
toxomiR®
toxomiR® – A Comprehensive Biomarker Panel for Drug-Induced and Environmental Toxicity
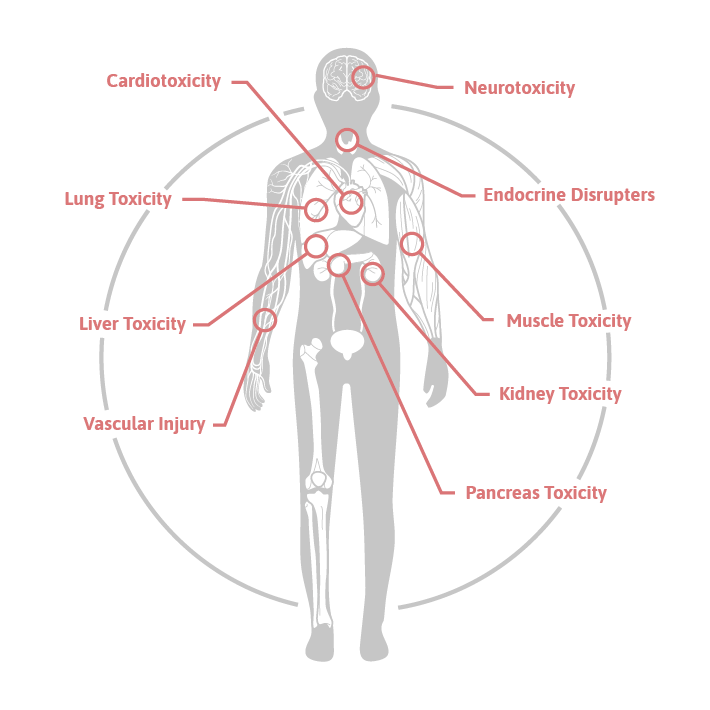
Toxicity remains a critical challenge in drug development and environmental safety assessments, necessitating reliable, translatable biomarkers. toxomiR® is a unique panel of tissue- and cell-type-specific microRNAs that enables the parallel detection of organ injury across eight different organs: liver, kidney, central nervous system, heart, muscle, lung, pancreas, and vasculature.
toxomiR® levels can be measured from serum, plasma, and urine in both preclinical and clinical studies, providing a non-invasive, translatable approach to detect organ toxicity. Importantly, the magnitude of toxomiR changes correlates with histopathological tissue damage, offering a powerful tool for early toxicity assessment and safety monitoring.
This review published by our scientists gives detailed insight into the clinical utility of circulating microRNAs as toxicity biomarkers.
benefits
Flexibility: the toxomiR® panel is available as an in-house service from TAmiRNA. Customers can select between the full toxomiR® panel covering 8 organ systems or customized panels for selected organs.
Accessibility: Non-invasive screening of toxic effects using serum, plasma, or urine.
Translatability: High sequence conservation of microRNA biomarkers enables rapid cross-species translation from pre-clinical to clinical studies.
Performance: toxomiRs are sensitive and specific biomarkers suitable for diagnosing tissue damage in the liver, pancreas, heart, skeletal muscle, vasculature, kidney, lung, and brain.
Feasibility: our toxomiR® analysis services provide a fast, cost-effective solution for leveraging microRNA biomarkers in preclinical and clinical toxicity evaluations, enabling efficient and reliable organ injury assessment.
service requirements
- Frozen serum, plasma, or urine samples.
- 25 – 200 µl input volume required.
- the toxomiR miRNA biomarker sequences are highly conserved, supporting the analysis of human, mouse, rat, dog, pig, and NHP samples.
intended use
toxomiRs® can be used in pre-clinical and clinical phases of drug safety assessment. The microRNAs panel enable early and reliable detection as well as monitoring of adverse events.
The toxomiR® panel can be used:
- To understand toxic mechanisms in liver, kidney, central nervous system, heart, muscle, lung, pancreas, vascular injury, and due to endocrine disruption
- For identifying and monitoring safety risks in drug development
- For early detection of tissue injury
service description
- The toxomiR® panel is only available as in-house service
- 200 µL serum and plasma are recommended (lower volumes can be used but require pre-testing)
- Deep-frozen (-70°C or lower) serum and plasma samples can be used
- 19 toxomiRs® and 5 quality controls are measured per sample
- Ready-to-use data for presentation and publication will be provided
deliverables
- Standardized analysis of qPCR data
- Quality control: inspect amplification curves, melting temperature, spike-in and hemolysis controls
- Ready-to-use data for presentation and publication will be provided
- Post project consultations to discuss next steps are included
- Customization is possible: upon request the analysis can be targeted to a specific set of microRNAs/tissue
price list
| sample number | price/sample |
|---|---|
| n<=48 | € 275.– |
| n<=96 | € 194.– |
| n<=154 | € 175.– |
| n<=192 | € 156.– |
| n>192 | € 147.– |











